Evaluation of the paraspinal muscles mophology using magnetic resonance imaging in adult patients with nonspecific chronic low back pain in Port Harcourt Rivers state Nigeria
Evaluación de la morfología de los músculos paraespinales mediante resonancia magnética en pacientes adultos con lumbalgia crónica inespecífica en Port Harcourt, estado de Rivers, Nigeria
MaryJane Amadi1, Nengi Alazigha1, Olukunmi Ijeruh1, Rufus Abam1, Awajimijan Nathaniel Mbaba1, Michael Promise Ogolodom2 *
1Department of Radiology, Rivers State University Teaching Hospital, Port Harcourt, Nigeria.
2Department of Radiography, Faculty of Basic Medical Sciences, College of Medical Sciences, Rivers State University, Port Harcourt, Nigeria.
![]()
Received: 16-02-2024 Revised: 21-08-2024 Accepted: 05-12-2024 Published: 06-12-2024
How to Cite: Amadi M, Alazigha N, Ijeruh O, Abam R, Mbaba AN, Ogolodom MP. Evaluation of the paraspinal muscles mophology using magnetic resonance imaging in adult patients with nonspecific chronic low back pain in Port Harcourt Rivers state Nigeria. Interamerican Journal of Health Sciences. 2024; 4:256. https://doi.org/10.59471/ijhsc2024256
ABSTRACT
Introduction: nonspecific chronic low back pain (CLBP) is a major public health concern globally and the leading cause of musculoskeletal disability, affecting mainly adults in their productive years with a high economic impact in the society.
Objective: this study was designed to evaluate the changes in the paraspinal muscles (lumbar multifidus) morphology using data obtained from MRI of the lumbosacral spine in adult participants with non-specific chronic low back pain.
Method: this was a cross-sectional study, involving 200 adult participants with non-specific chronic low back pain undergoing MRI of the lumbosacral spine at the Department of Radiology, Rivers State University Teaching Hospital (RSUTH), Port Harcourt, Nigeria, during a six months period. Data recorded include lumbar multifidus muscle (LMM) morphology and lumbar spine pathologies
Results: the LMM CSA for L1, L2, L3, L4 and L5 were 2,24±0,84, 3,59±1,15, 5,18±1,60, 6,66±1,75 and 7,27±2,62 respectively. The frequency distribution of LMM fatty infiltration in percentage showed Grade 0 was highest at L1 (86,5 %, n= 173) and lowest at L5 (6,5 %, n=13); Grade 1 was highest at L4 (73 %, n=146) and lowest at L1 (13,5 %, n=2) and Grade 2 was highest at L5 (22,5 %, n=45) and lowest at L1 (0,0 %, n=0). The lumbar pathologies noted were 200 (100 %) disc abnormalities, while 178 (89,0 %) had neural foraminal narrowing, nerve root compression and spinal canal stenosis each. Facet joint arthrosis and spondylosis were 177 (88,5 %) and 176 (88,0 %) respectively.
Conclusion: dysfunctional LMM was observed to increase with advancing age, severe in females and individuals with elevated BMI and cuts across various occupations. Measures aimed at improving and maintaining the quality of the LMM should be encouraged, as this will help in reducing the prevalence of nonspecific CLBP.
Keywords
Degenerative Changes; Lower Back Pain; MRI.
RESUMEN
Introducción: el dolor lumbar crónico inespecífico (DLC) es un importante problema de salud pública a nivel mundial y la principal causa de discapacidad musculoesquelética, afectando principalmente a adultos en edad productiva con un alto impacto económico en la sociedad.
Objetivo: este estudio se diseñó para evaluar los cambios en la morfología de los músculos paraespinales (multífido lumbar) mediante datos obtenidos de la RM de la columna lumbosacra en participantes adultos con lumbalgia crónica inespecífica.
Método: se trató de un estudio transversal en el que participaron 200 adultos con lumbalgia crónica inespecífica a los que se practicó una RM de la columna lumbosacra en el Departamento de Radiología del Hospital Universitario del Estado de Rivers (RSUTH), Port Harcourt, Nigeria, durante un periodo de seis meses. Los datos registrados incluyen la morfología del músculo multifidus lumbar (MML) y las patologías de la columna lumbar.
Resultados: el LMM CSA para L1, L2, L3, L4 y L5 fueron 2,24±0,84, 3,59±1,15, 5,18±1,60, 6,66±1,75 y 7,27±2,62 respectivamente. La distribución porcentual de la frecuencia de la infiltración grasa del LMM mostró que el grado 0 era el más alto en L1 (86,5 %, n= 173) y el más bajo en L5 (6,5 %, n=13); el grado 1 era el más alto en L4 (73 %, n=146) y el más bajo en L1 (13,5 %, n=2) y el grado 2 era el más alto en L5 (22,5 %, n=45) y el más bajo en L1 (0,0 %, n=0). Las patologías lumbares observadas fueron 200 (100 %) anomalías discales, mientras que 178 (89,0 %) presentaban estrechamiento de la foraminal neural, compresión de la raíz nerviosa y estenosis del canal espinal cada una. La artrosis de la articulación facetaria y la espondilosis eran 177 (88,5 %) y 176 (88,0 %) respectivamente.
Conclusiones: se observó que la LMM disfuncional aumentaba con la edad, era grave en las mujeres y en los individuos con IMC elevado y afectaba a varias ocupaciones. Deben fomentarse las medidas encaminadas a mejorar y mantener la calidad del MLM, ya que ello contribuirá a reducir la prevalencia de la dolor lumbar inespecífico.
Palabras clave
Cambios Degenerativos; Lumbalgia; IRM.
INTRODUCTION
Low back pain (LBP) refers to pain, muscle tension, or stiffness at the back that is localized below the costal margin and above the inferior gluteal folds, with or without sciatica/leg pain.(1) It is classified based on duration of the symptoms as acute (when the symptoms last less than 3 months) or chronic (when the symptoms last more than 3 months). Based on the etiology of symptoms, it is also classified as specific when there is a known causative factor or non-specific when there is no known cause.(2) According to the Global Burden of Disease (GBD) 2015 study, LBP is the leading cause of disability worldwide, accounting for 815 Years Lived with Disability (YLD) per 100,000 populations, with this value representing a 17,2 % increase since 2005.(3) The greatest burden has been observed in low to medium income nations like those in the Sub-Saharan Africa, where health care resources are limited in addressing this problem besides priorities such as infectious diseases (malaria and tuberculosis).(4,5) Chronic low back pain (CLBP) comprises 10-20 % of LBP,(6) with 80-90 % of CLBP being non-specific and intractable,(2) relapsing and causing multiple episodes of LBP after the initial attack, resulting in frequent hospital visits and absenteeism from work.(6,7)
CLBP is a condition predominantly affecting individuals in their productive years (40-59 years)(8) with resultant loss of finances, frequent hospital visits, poor quality of life, further exacerbating poverty.(7,9) Thus, the effect of non-specific CLBP on wellbeing, health-related quality of life and functioning in this age group is substantial, with the indirect cost due to productivity loss representing a large proportion of the overall cost which can represent 50 – 89 % of the total cost.(10)
Reports have stated that there is an estimated global lifetime prevalence of 70-80 %, one year prevalence of 15-45 % and an averaged point prevalence of 30 % among the general population.(9) In Western countries’ settings, evidence suggests LBP affects 40-60 % of working adults and adversely impacts the quality of life, frequently on a daily basis.(11) A systematic review by Morris et al investigating the prevalence of low back pain in Africa revealed that the lifetime, one-year and point prevalence of low back pain among African populations was substantially higher than the revealed global LBP prevalence estimates.(9) He found that the point prevalence of low back pain among African countries was 39 %, which was higher than the 28,8 % reported among adult Americans in 2013. In Cameroon, the prevalence of CLBP was 19,1 % among patients presenting for rheumatology consultations during 2004 to 2013 at the Douala General Hospital.(12) In Nigeria, a systematic review done by Bello et al showed that the 12-month prevalence ranged from 32,5 % to 73,53 %.(13) In this review by Bello et al, five studies reported point prevalence of LBP and it ranged from 14,7 % to 59,7 %, two studies reported lifetime prevalence of LBP as 45,5 % and 58 % while one study reported a 7-day prevalence of 11,5 %. From the above study, the lifetime prevalence of CLBP in Africa is comparable to the rates in the Western societies, and this also will impact on the quality of life in Africa as seen in Western societies.(11)The burden of CLBP in Nigeria is extremely greater in rural communities which have a one year prevalence rate ranging between 70 % - 85 %, compared to urban communities with a prevalence rate of 39 %.(14,15) This difference is due to higher literacy rates and paid employment which is associated with differential access to infrastructure and amenities in the urban communities as well as unwillingness of health professionals to travel to rural areas to treat patients with disability.(15) The prognosis of chronic low back pain is therefore worse in the rural communities as they have limited access to health professionals, infrastructure and amenities, thus increasing disability and poverty. Nonspecific chronic low back pain is said to affect males and females alike, however, more males (73,5 %) are affected than females (71,0 %), thus, worsening the impact of this disease condition by imposing considerable economic burden on the family and society as males are mostly the bread winners in these countries, contributing about 70,3 % to the labor force.(14,16)
Studies have shown that a high prevalence of overweight and obesity contribute to the burden of non-specific chronic low back pain in the population.(17) Currently, 1 in 5 adults are morbidly obese in America while the trend is on the rise across urban settings in several low- and middle-income countries with an estimated 21 million and 12 million overweight and obese persons in the Nigerian population aged 15 years or more in 2020 and higher among women than men, which may in part be due to widespread sedentary lifestyles, and a surge in processed food outlets, largely reflective of a trend across many African settings.(18)
The prevalence of CLBP is noted to be predominant among the working class adults,(14) in their productive years (40-59 years),(8) highest amongst the employee whose job requires prolonged sitting or standing, intensive use of computers and other technologies at work and at home, physically demanding occupations causing repetitive strain to the back muscles from poor ergonomics and lifting techniques, with associated significant levels of disability, producing restrictions in usual activity, inability to work, presenteeism, absenteeism, colossal loss of workforce hours and socioeconomic burden.(7,9,19)
The lumbar paraspinal muscles are not often mentioned in studies of the spine for CLBP. Nonetheless, in recent times, attention has shifted towards the paraspinal muscles, as variations in paraspinal muscle morphology have been observed in patients with LBP.(20) It has been reported that specific management tailored at these muscles have been observed to decrease pain and improve the stability of the spine.(21) Hence the paraspinal muscles need to be considered in association with the clinical presentation and other spine abnormalities seen on imaging as they are the key stabilizers of the back. The aim of this study is to evaluate the lumbar paraspinal stabilizing muscles [particularly lumbar multifidus muscle (LMM)] morphology and other lumbar spine degenerative changes in patients with non-specific CLBP in our setting using magnetic resonance imaging (MRI).
METHOD
Study design
This was a cross-sectional study of the participants who presented to the Radiology Department of Rivers State University Teaching Hospital (RSUTH) with complaints of non-specific CLBP for MRI of the lumbosacral spine. Convenient non-random sampling method was used.
Study site and duration of study
This study was carried out in the Radiology Department of Rivers State University Teaching Hospital (RSUTH), Port-Harcourt. The services of the department meet the radio-diagnostic needs of the clinical departments of the hospital in addition to referrals from other hospitals within Rivers State and the Niger Delta region of Nigeria. The duration of this study spanned from 26th August 2020 to 15th January 2021.
Ethical consideration and study population
Ethical approval (RSHMB/RSHREC/11,19/Vol.7/040) was sought and approval obtained from the Research and Ethics Committee of the Rivers State University Teaching Hospital, Port Harcourt prior to the commencement of the study. Participation in the study was voluntary and informed consent was obtained prior to inclusion in this study.
The study population consists of patients referred to the Department of Radiology from various Clinical Departments of RSUTH as well as other referral centers within and around Port Harcourt for lumbar spine MRI scan within the period of this study.
Sample size determination and sampling technique
The sample size for this study was determined using the formula.
n =
Where n = desired sample size
N = population of study (400)
e = accepted error limit (0,05)
n = 200
Therefore, 200 participants were recruited into the study. The participants were randomly selected into the study.
Study procedure
Consent was obtained with signatures on the form and confidentiality maintained for those who met the inclusion criteria. Research participants’ biodata and demographic data such as age, sex, weight, height, marital status, and occupation was gotten using participants’ data sheet. The occupational status of the participants was classified as, Professionals, Managers, Non-manual skilled, Manual skilled, Semi-skilled and Unskilled or unemployed.(17) The body mass index (BMI) was calculated with the formula. BMI=weight (kg)/height2 (m2). Weight was estimated with a weighing scale in kilogram (kg) and height with a meter rule in meters (m). BMI was categorized based on WHO criteria as underweight (< 18,5 kg/m2), normal weight (18,5-24,9 kg/m2), overweight (25-29,9 kg/m2), and obese (>30 kg/m2).
Imaging technique
Images were acquired using a GE® Medical System Signa Creator 1,5 Tesla superconducting magnet bore size 60cm January 2018 MRI-scanner from Radiology department RSUTH for the different participants. Basic imaging was done using conventional spin echo pulse sequences. Images were acquired in axial, sagittal and coronal planes. MR Images of the lumbosacral spine of the participants that were acquired were sent to the doctors’ workstation/computer system for interpretation which was done by the researcher, a resident and an experienced consultant to arrive at a consensus report on each study in order to ensure the accuracy of the results that were obtained. Both normal findings as well as abnormal findings seen were documented on a datasheet.
Data analysis
Data was entered into a computer spread sheet after recording in the participants’ data sheet.
Statistical analysis was performed using Statistical Package for Social Sciences, SPSS version 21.
Data was represented as mean and standard deviation for continuous variables while frequency distribution was utilized for categorical variables. The continuous variables were compared using the student t-test. Also, Analysis of Variance (ANOVA), statistic was used as appropriate to compare variables. Results were presented as mean ± standard deviation, percentages, tables, and charts as appropriate. A p-value of <0,05 was considered significant.
RESULTS
The participants’ age ranged from 24 to 65 years with a mean age of 48,39±11,32 and 48,18±11,62 years for males and females respectively. Amongst the study population, 121 subjects (60,5 %) were males while 79 subjects (39,5 %) were females giving a male to female ratio of 1,53:1 (table 1).
|
Table 1. Comparison of mean age by sex of adult participants with non-specific CLBP |
||||
|
Variable |
Male n=121 (60,5 %) Mean ± SD |
Female n=79 (39,5 %) Mean ± SD |
t |
p-value |
|
Age in year |
48,39±11,32 |
48,18±11,62 |
0,128 |
0,899 |
|
SD – Standard deviation |
||||
Of the study population, 15 subjects (7,5 %) were single, 178 subjects (89,0 %) were married, 4 subjects (2,0 %) were separated/divorced, and 3 subjects (1,5 %) were widowed. The majority (37,5 %) of the participants was professionals and the least (2,5 %) were unskilled workers (table 2).
|
Table 2. Socio-demographic characteristics of adult participants with non-specific CLBP |
|||
|
Variables |
Male n ( %) |
Female n ( %) |
Total n ( %) |
|
Age category |
|||
|
≤25 years |
4 (3,3) |
0 (0,0) |
4 (2,0) |
|
26 – 35 years |
6 (5,0) |
13 (16,4) |
19 (9,5) |
|
36 – 45 years |
52 (43,0) |
19 (15,7) |
71 (35,5) |
|
46 – 55years |
20 (16,5) |
22 (27,8) |
42 (21,0) |
|
>55 years |
39 (32,2) |
25 (31,6) |
64 (32,0) |
|
Marital status |
|||
|
Single |
7 (5,8) |
8 (10,1) |
15 (37,5) |
|
Married |
111 (91,7) |
67 (84,8) |
178 (89,0) |
|
Separated/Divorced |
2 (1,6) |
2 (2,5) |
4 (2,0) |
|
Widowed |
1 (0,8) |
2 (2,5) |
3 (1,5) |
|
Occupational status |
|||
|
Professional |
50 (41,3) |
25 (31,6) |
75 (37,5) |
|
Managers |
23 (19,0) |
27 (34,2) |
50 (25,0) |
|
Non-manual skilled |
27 (22,3) |
13 (16,4) |
40 (20,0) |
|
Manual skilled |
6 (5,0) |
6 (7,6) |
12 (6,0) |
|
Semi-skilled |
8 (6,6) |
1 (1,3) |
9 (4,5) |
|
Unskilled |
2 (1,7) |
3 (3,8) |
5 (2,5) |
|
Unemployed/Student |
5 (4,1) |
4 (5,1) |
9 (4,5) |
|
Tribe |
|||
|
Igbo |
21 (17,4) |
18 (22,8) |
39 (19,5) |
|
Yoruba |
6 (5,0) |
4 (5,1) |
10 (5,0) |
|
Ikwerre |
32(26,4) |
15 (19,0) |
47 (23,5) |
|
Ogoni |
7 (5,8) |
4 (5,1) |
11 (5,5) |
|
Kalabari/Okirika |
14 (11,6) |
17 (21,6) |
31 (15,5) |
|
Urhobo |
2 (1,7) |
3 (3,8) |
5 (2,5) |
|
Ibibio/Efik |
10 (8,3) |
3 (3,8) |
13 (6,5) |
|
Others |
29 (24,0) |
15 (19,0) |
44(22,0) |
The mean BMI was 26,42±3,44 kg/m2 (overweight), among age category 46–55 years with 60 (49,6 %) males and 41 (51,9 %) females. The mean BMI for males was 26,43±3,37 kg/m2 and for females 26,40±3,56 kg/m2 (p=0,949) as seen in table 3. One participant (0,8 %) was underweight (BMI <18,5 kg/m2) in age category <25 years, 16 (84,2 %) had normal weight (BMI 18,5–24,9 kg/m 2) in age category 26–35 years, 25 (59,5 %) were overweight (BMI 25–29,9 kg/m 2) in age category 46–55 years and 15(23,4 %) were obese (BMI > 30 kg/m 2) in age category >55 years (table 3).
|
Table 3. Comparison of mean BMI by sex of adult participants with non-specific CLBP |
|||||
|
Variables |
BMI categories |
Total n (%) |
|||
|
Underweight n (%) |
Normal n (%) |
Over-weight n (%) |
Obese n (%) |
||
|
Age category |
|||||
|
≤25 years |
1 (25,0) |
2 (50,0) |
1 (25,0) |
0 (0,0) |
4 (100,0) |
|
26 – 35 years |
0 (0,0) |
16 (84,2) |
2 (10,5) |
1 (5,3) |
19 (100,0) |
|
36 – 45 years |
0 (0,0) |
22 (31,0) |
38 (53,5) |
11 (15,5) |
71 (100,0) |
|
46 – 55years |
0 (0,0) |
13 (31,0) |
25 (59,5) |
4 (9,5) |
42 (100,0) |
|
>55 years |
0 (0,0) |
14 (21,9) |
35 (54,7) |
15 (23,4) |
64 (100,0) |
|
Fisher’s exact test = 79,736; p-value = 0,0001* |
|||||
|
Sex |
|||||
|
Male |
1 (0,8) |
40 (33,1) |
60 (49,6) |
20 (16,5) |
121 (100,0) |
|
Female |
0 (0,0) |
27 (34,2) |
41 (51,9) |
11 (13,9) |
79 (100,0) |
|
Fisher’s exact test = 0,880; p-value = 0,942 |
|||||
|
*Statistically significant (p<0,05) |
|||||
Among the male population, 1 (0,8 %) was underweight, 40 (33,1 %) had normal BMI, 60 (49,6 %) were overweight, and 20 (16,5 %) were obese. No female was underweight (0,0 %), 27(34,2 %) had normal BMI, 41 (51,9 %) were overweight and 11 (13,9 %) were obese (figure 1).
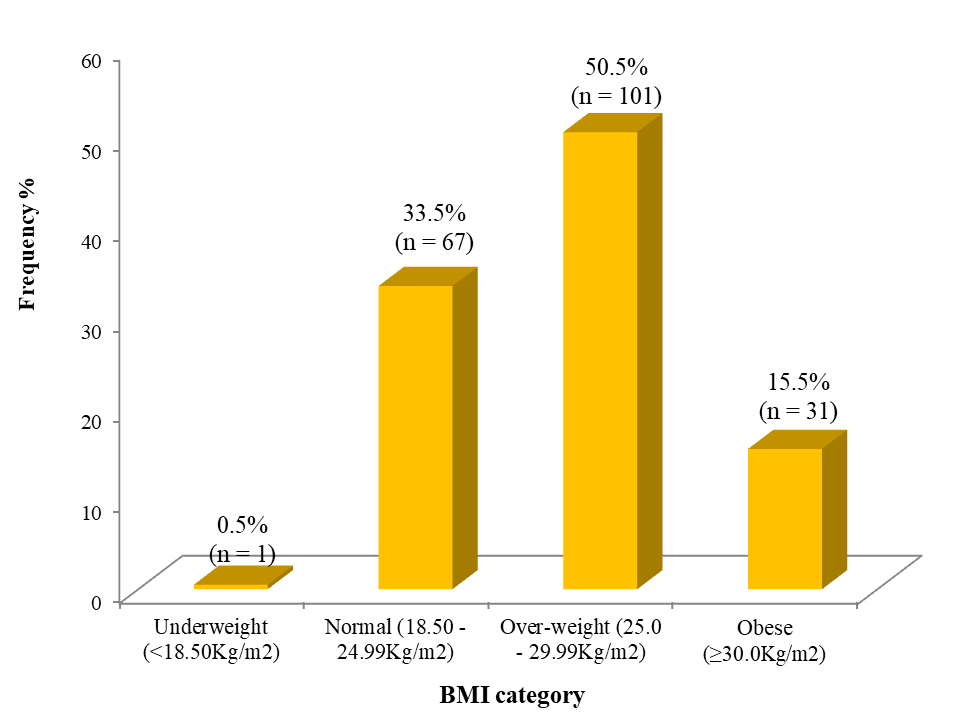
Figure 1. BMI categories of adult participants with non-specific CLBP
The mean and standard deviation values for cross-sectional areas of L1, L2 and L3 are 2,24±0,84, 3,59±1,15 and 5,18±1,60 respectively (table 4).
|
Table 4. Summary of LMM CSA in adult participants with non-specific CLBP |
|||
|
Lumbar segments |
Cross Sectional Area |
Median |
|
|
Range |
Mean ± SD |
||
|
L1 |
1,00 – 5,70 |
2,24±0,84 |
2,10 |
|
L2 |
1,50 – 8,20 |
3,59±1,15 |
3,40 |
|
L3 |
2,50 – 12,60 |
5,18±1,60 |
5,00 |
|
L4 |
3,20 – 12,40 |
6,66±1,75 |
6,40 |
|
L5 |
1,10 – 14,70 |
7,27±2,62 |
7,10 |
|
SD – Standard deviation |
|||
Grade 0 fatty infiltration was predominantly seen in the upper lumbar spine as 86,5 % and 75,5 % of grade 0 fatty infiltration were concentrated at L1 and L2 lumbar levels respectively. Much lower percentages of grade 0 fatty infiltration were observed in the lower lumbar levels (table 5).
|
Table 5. Summary of LMM fatty infiltration composition in adult participants with non-specific CLBP |
||
|
|
LMM Fatty Infiltration Composition |
|
|
Lumbar segments |
Median |
Range |
|
L1 |
0 |
0 – 1 |
|
L2 |
0 |
0 – 2 |
|
L3 |
1 |
0 – 2 |
|
L4 |
1 |
0 – 2 |
|
L5 |
1 |
0 – 2 |
Grade 1 fatty infiltration was observed in the upper lumbar spine, however at 13,5 % and 24,0 % at L1 and L2 levels respectively. L3, L4 and L5 levels had grade 1 fatty infiltration of 64,0 %, 73,0 % and 71,0 % respectively. None of grade 2 fatty infiltration was observed at L1 lumbar level. L2 lumbar level had 0,5 % grade 2 fatty infiltrations while L3, L4 and L5 lumbar levels had 1,5 %, 13,0 % and 22,5 % respectively (figure 2).
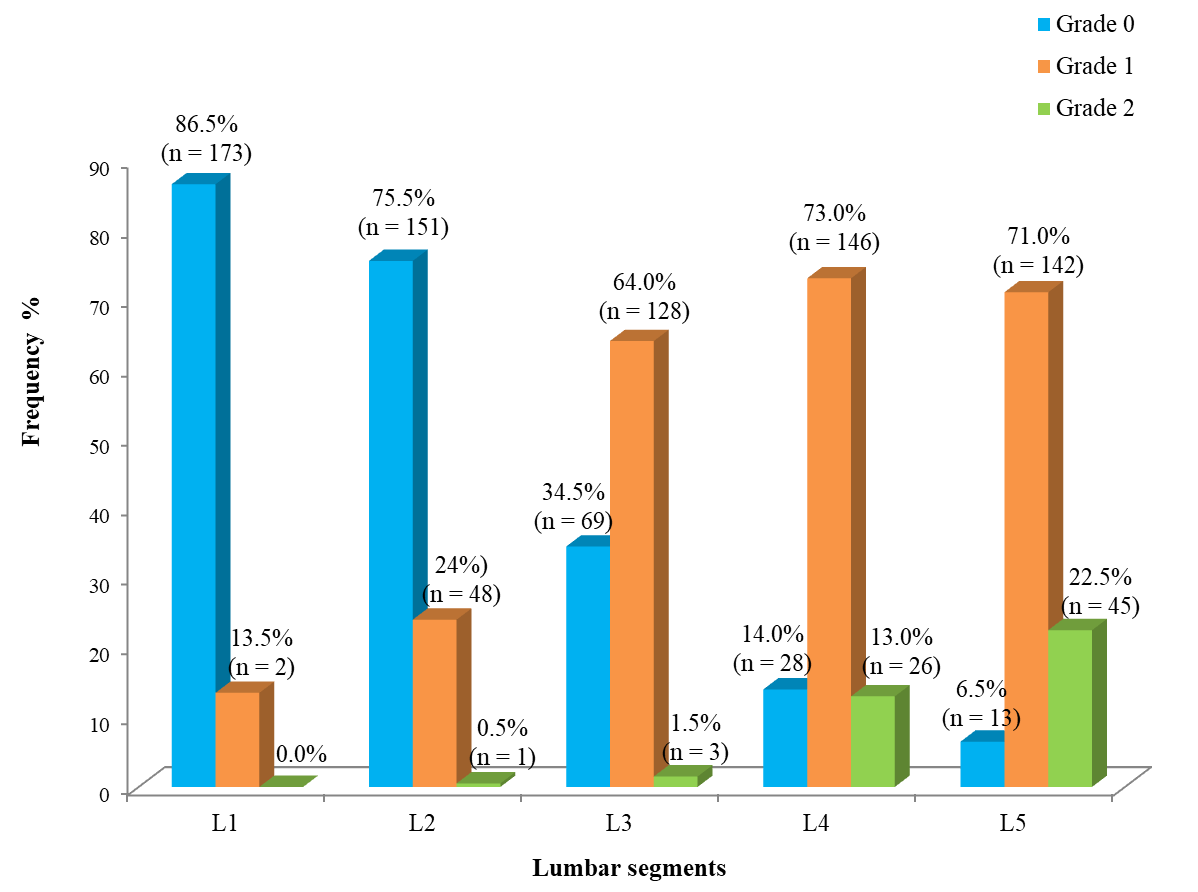
Figure 2. Distribution of LMM fatty infiltration grades according to the lumbar segments in adult participants with non-specific CLBP
With regards to pathologies across lumbar levels in adult participants with Non-Specific CLBP, at L1 level, the most frequent of these abnormalities were spondylosis, which was present in 56 participants (28,0 %), disc changes 45 (22,5 %), and facet joint arthrosis 32 (16,0 %). Neural foraminal narrowing, nerve root compression and spinal canal stenosis at 26 (13,0 %) each were documented. At L2 level, same trend of pathologies were observed however at a higher frequency than what was recorded at L1 level; 85 (42,5 %) spondylosis, 78 (39,0 %) disc changes, 78 (39,0 %), facet joint arthrosis 56 (28,0 %). Neural foraminal narrowing, nerve root compression and spinal canal stenosis had 57 (28,5 %) each. One hundred and fifty-five (155) participants (77,5 %) had disc abnormalities, 138 (69,0 %) spondylosis, and 126 (63 %) facet joint arthrosis. Neural foraminal narrowing, nerve root compression and spinal canal stenosis were 111 (55,5 %) each. At L4 level, disc changes were the commonest lumbar pathology amongst all the participants. The number of participants who had these lumbar pathologies noted were 200 (100 %) disc abnormalities, while 178 (89,0 %) had neural foraminal narrowing, nerve root compression and spinal canal stenosis each. Facet joint arthrosis and spondylosis were 177 (88,5 %) and 176 (88,0 %) respectively. At L5 level, disc abnormalities were 181(90,5 %) spondylosis 168(84 %), facet joint arthrosis 155 (77,5 %). One hundred and fifteen (115) that is (57,5 %) each for neural foraminal narrowing nerve root compression and spinal canal stenosis (table 6).
|
Table 6. Lumbar pathologies in adult participants with non-specific CLBP |
|||||
|
Lumbar pathologies |
L1 n ( %) |
L2 n ( %) |
L3 n ( %) |
L4 n ( %) |
L5 n ( %) |
|
Disc changes |
|||||
|
Present |
45 (22,5) |
78 (39,0) |
155 (77,5) |
200(100,0) |
181 (90,5) |
|
Absent |
155 (77,5) |
122 (61,0) |
45 (22,5) |
0 (0,0) |
19 (9,5) |
|
Facet joint |
|||||
|
Present |
32 (16,0) |
56 (28,0) |
126 (63,0) |
177 (88,5) |
155 (77,5) |
|
Absent |
168 (84,0) |
144 (72,0) |
74 (37,0) |
23 (11,5) |
45 (22,5) |
|
Neural foramen |
|||||
|
Present |
26 (13,0) |
57 (28,5) |
111 (55,5) |
178 (89,0) |
115 (57,5) |
|
Absent |
174 (87,0) |
143 (71,5) |
89 (44,5) |
22 (11,0) |
85 (42,5) |
|
Nerve root compression |
|||||
|
Present |
26 (13,0) |
57 (28,5) |
111 (55,5) |
178 (89,0) |
115 (87,5) |
|
Absent |
174 (87,0) |
143 (71,5) |
89 (44,5) |
22 (11,0) |
85 (42,5) |
|
Cord compression |
|||||
|
Present |
0 (0,0) |
0 (0,0) |
0 (0,0) |
0 (0,0) |
0 (0,0) |
|
Absent |
200 (100,0) |
200 (100,0) |
200 (100,0) |
200 (100,0) |
200 (100,0) |
|
Spinal canal stenosis |
|||||
|
Present |
26 (13,0) |
57 (28,5) |
111 (55,5) |
178 (89,0) |
115 (57,5) |
|
Absent |
174 (87,0) |
143 (71,5) |
89 (44,5) |
22 (11,0) |
85 (42,5) |
|
Spondylosis |
|||||
|
Present |
56 (28,0) |
85 (42,5) |
138 (69,0) |
176 (88,0) |
168 (84,0) |
|
Absent |
144 (72,0) |
115 (87,5) |
62 (31,0) |
24 (12,0) |
32 (16,0) |
|
Spondylolisthesis |
|||||
|
Present |
0 (0,0) |
0 (0,0) |
0 (0,0) |
0 (0,0) |
0 (0,0) |
|
Absent |
200 (100,0) |
200 (100,0) |
200 (100,0) |
200 (100,0) |
200 (100,0) |
There difference in mean LMM CSA between age categories of all the participants was significant for L2 (p= 0,01), L3 (p=0,002), L4 (p= 0,001) and L5 (p=0,001), however the difference in mean LMM CSA between age categories at L1 level was not statistically significant (p=0,106) (table 7).
|
Table 7. Age-related changes in LMM CSA in adult participants with non-specific CLBP |
|||||
|
Age Category |
L1 Mean ± SD |
L2 Mean ± SD |
L3 Mean ± SD |
L4 Mean ± SD |
L5 Mean ± SD |
|
≤25 years |
1,90±0,64 |
3,95±1,19 |
6,05±1,64 |
7,80±2,38 |
8,73±3,26 |
|
26 – 35 years |
2,36±1,10 |
3,78±1,48 |
5,07±1,40 |
6,55±1,76 |
7,52±2,14 |
|
36 – 45 years |
2,43±0,92 |
3,98±1,13 |
5,74±1,68 |
7,30±1,72 |
8,15±3,10 |
|
46 – 55 years |
2,17±0,69 |
3,17±0,75 |
4,89±1,73 |
6,27±1,62 |
6,23±2,13 |
|
>55 years |
2,06±0,77 |
3,36±1,15 |
4,71±1,29 |
6,16±1,61 |
6,81±2,08 |
|
ANOVA p-value |
1,937 0,106 |
4,592 0,001* |
4,461 0,002* |
5,019 0,001* |
4,823 0,001* |
|
*Statistically significant (p<0,05); SD – Standard deviation |
|||||
DISCUSSION
This study evaluated LMM morphology with MRI in adult participants with nonspecific CLBP. The mean age of participants in the study was 48,39±11,32 and 48,18±11,62 years for males and females, respectively. The age distribution of the participants was between 24 – 65 years with the largest subset 35,5 % (71) of participants between the 4th and 5th decade similar to the pattern reported by Omoke and Amaraegbulam(22) in a study done in Enugu, Nigeria where the mean age was 45,8 years. This is in line with a study done by Ahidjo et al.(23) in a major teaching hospital in Nigeria which concluded that the age bracket of patients with CLBP is between 41 – 50 years. However, in a prior study done in Port Harcourt, a mean age of 51 years was recorded,(17) while another study done at two imaging centers in the South West and North Central Nigeria, the mean age was 52,5 years.(24) Comparing the mean ages of the various studies, it can be seen that the mean age of the population developing nonspecific CLBP is gradually reducing as the younger generation are becoming the working class, as the occupational sector is characterized by the demand for better skilled labor, which can be related to the important increase in CLPB among younger and more educated individuals. This calls for urgent attention as more people in the population will eventually develop this condition as the population ages and this in turn will affect the society, thus corroborating the negative effects of CLBP on productivity and economy as pointed out in prior studies.(7,25) The finding of this study is at variance with the results obtained by Adegoke et al.(26) who studied adolescents (age range 10 – 19 years) and stated that low back pain is milder and transient in this age group because degenerative changes take time to set in, such that these individuals are able to recover faster, thus not serious enough to cause disability. This disparity is probably due to the age of the population Adegoke et al.(26) studied, as their study was done on adolescents (age range 10 – 19 years) while the index study was done on subjects between 18 – 65 years. Although the younger age group (< 40 years) has been observed to come down with low back pain, it is not as severe and common as it is in those above 40 years.(25) The back pain the younger age group develop is usually less than a month and can be described as mild and transient, probably due to a larger LMM CSA. As such, may not be serious enough to cause disability.(26) In the current study, there was an association between increasing age and the development of nonspecific CLBP in adults and this could likely be due to the normal aging process, which as the population ages, the various effects of wear and tear become more pronounced. This is not surprising as research has shown that people have the expectation that health deteriorates with ageing.(15)
The index study showed a male preponderance in relation to females, thus agreeing with Iyidobi et al, who reported male preponderance in their study.(27) This could be due to the fact that males are more likely to be involved in more vigorous physical activities than their female counterparts, working longer hours so as to increase their earnings in order to improve their standard of living and provide for their families in order to fulfil their gender role.(15) Conversely, Omoke and Amaraegbulam,(22) as well as Adekanmi et al(25) in their respective studies recorded female preponderance. Likewise Awosan et al(28) and Abdulmujeeb et al(29) in their various studies noted female preponderance which is at variance with this study. This disparity could be due to differences in population studied, as well as dissatisfaction of males with conventional health care services due to failure of the pain to be cured and bureaucracy of the hospital services, that they have adopted coping strategies to deal with the pain. More studies are hereby needed to elucidate this difference.
Also, more of the study participants were professionals, in keeping with a prior study done in Port Harcourt.(17) This is probably due to intensive use of computers and related technologies that result in prolonged sitting both in the workplace and at home thus increasing the axial loading of the spine, and a higher income level among this category to afford the cost of MRI study. It was noted that the manual skilled, semi-skilled and unskilled workers had a higher percentage of the various abnormalities This could be due to the poor knowledge of correct posture/lifting techniques, and these participants are likely to be involved in more vigorous physical activities, as well as working longer hours so as to increase their earnings. This finding is consistent with the observations noted by other researchers.(15)
A mean BMI of 26,42+/-3,44 kg/m2 was noted in the study participants, which according to the WHO criteria for BMI classification is overweight (BMI 25 - 29,9 kg/m2), among age category 46 – 55 years with 60 (49,6 %) males and 41 (51,9 %) females. The highest frequency 15 (23,4 %) of obese participants fell within the sixth decade. This is in line with a previous study done in Port Harcourt where the study participants were overweight with a mean BMI of 29,5+/-5,2 kg/m2 and the highest frequency of obese participants was in the sixth decade.(17) This finding suggests that physical loading of the spine in the form of elevated BMI may biomechanically contribute to the development of pain. In addition, males had marginally higher mean BMI than females with a mean of 26,43 ± 3,37 versus 26,40 ± 3,56 respectively (P = 0,949). This is however at variance with other studies that showed the mean BMI to be higher in females than in males.(17) This difference could be due to increasing consciousness of females in managing their body weight.
In the present study the mean LMM CSA was noted to gradually increase in size up to the 4th and 5th decade where it peaks and thereafter starts declining in size across the lumbar levels in line with a prior longitudinal study.(30) With aging, levels of anabolic hormones, such as testosterone, growth hormone, and insulin-like growth factor (IGF)-1 decrease and that of catabolic agents like interleukin 6 (IL-6) increases, contributing to muscle wasting among elderly individuals.(31) This decline in LMM CSA was more significant at L4- L5 and L5-S1 levels than at other levels. This could be associated with substantial degenerative changes occurring more in the lower lumbar levels due to increase mobility of this region.(21) However, Crawford et al reported a different trend in age-related muscle CSA change, where multifidus and erector spinae volume was age-independent in 80 healthy adult volunteers using MRI.(32) The inconsistency of results between studies may be related to methodologic differences in the measurement methods (CT versus MRI) and parameters (CSA versus volume), targeted muscle groups, and study sample population. The severity of LMM CSA atrophy and advancing decades were noted to be directly proportional. LMM atrophy was assessed in axial sections from L1 to L5 levels, in order to achieve a true and standardized multilevel evaluation of the LMM as suggested by Urrutia et al.(33) in an earlier study .
This study noticed a significantly higher mean LMM CSA across all the lumbar levels. This is as documented by Rummens et al.(34) that found the LMM CSA to increase in a caudal progression. This is probably due to the increased load the lower lumbar levels have to bear as well as the high mobility in this region. Studies have shown LMM CSA to be reduced in participants with CLBP due to disuse or lack of exercise.(31) Some studies have suggested that paraspinal muscles are smaller in participants with chronic LBP than in control participants and on the symptomatic side of participants with chronic unilateral CLBP as reported by Rummens et al.(34) in their literature review of chronic low back pain as a whole.
Rummens et al.(34) and Evanson et al.(35) in their review of some studies also noted no significant difference in LMM CSA between those with and without CLBP. This may be due to the fact that they conducted their study among athletes that are elite ballroom dancers, that have specialized dance routines and training programs, which seemed to have influenced LMM size regardless of the presence of back pain. This study demonstrated that the mean LMM CSA in women is significantly smaller than the mean LMM CSA in men. This may be due to males having a larger body mass compared to females, agreeing with a previous study that noticed these changes to be pronounced in females.(36) In Rummens et al.(34) review, it was found that male LMM CSA was greater when compared to that of females, prior to being normalized by body mass, with no significant differences in symmetry between genders. Also, in Rummens et al.(34) review, two studies recorded no significant difference in symmetry of CSA between sexes in an asymptomatic population or in patients with CLBP. This study also demonstrated that BMI affected the LMM CSA of the participants because it was noted that the size of the LMM CSA in those with higher BMI was more than those with lower or normal BMI. This increase in size is due to the increased accumulation of adipose tissue within the muscle fibers. Other studies noted a similar finding.(37)
In this study, median rank LMM fatty infiltration was noted to be more in the participants >40 years than in participants <40 years. The LMM fatty infiltration rate increases with age as a result of human type 1 muscles fibers accumulating fat with age leading to dysfunction and disability(37) In a prior study done in a younger age group than in this study population, pain was noted to be transient with complete recovery probably due to reduced accumulation of fat within the muscle.(26) Thus, it takes a longer time for LMM fatty infiltration to occur. LMM fatty infiltration was noted in all the lumbar levels, and it increased gradually in severity across the lumbar levels. These findings were also seen by Vohra et al.(38) who showed the presence of LMM fatty infiltration at all lumbar levels and the fat content of the muscle gradually increased from L1-L5 level. The increase in LMM fatty infiltration was worse at the lower lumbar levels. This could be due to the increased rate of degenerative changes leading to atrophy that occur in this region. This agrees with previous studies which also noted atrophy to occur prior to LMM fatty infiltration.(39,40) LMM fatty infiltration was noted to be more in females compared to males amongst the study participants in the present study, consistent with the report from a previous study.(41) The effects of female hormones during pregnancies and childbirths might explain this difference to some extent, as gestational weight gain is a known physiological phenomenon.(41) Also, the effect of postmenopausal hormone withdrawal could play a role.(42) Furthermore, type 1 fibers or slow twitch oxidative fibers which are more in females, tend to accumulate more lipid with age in humans than type 2 fibers or fast twitch oxidative fibers.(39) This present study therefore positively correlates that LMM fatty infiltration is commoner in adults, strongly associated with CLBP, worse in the lower lumbar levels and marked in females.(25)
CONCLUSION
Dysfunctional LMM with smaller CSA and increased fatty infiltration is the common denominator in adult participants with nonspecific CLBP in our environment, affecting all the lumbar levels and can be detected on MRI where it is found commonly in the lower lumbar levels. A significant relationship was found between LMM CSA and the occurrence of lumbar pathologies. In participants with lumbar pathologies, their mean LMM CSA was found to be significantly smaller than in those without the particular pathologies. Also, a significant relationship was noted between LMM fatty infiltration and the occurrence of lumbar pathologies, as the mean ranking LMM fatty infiltration was significantly higher among participants with lumbar pathologies in comparison with those without the particular pathologies. Similarly, a significant relationship was noted between elevated BMI and occurrence of lumbar pathologies as the percentage of lumbar pathologies was higher among participants with elevated BMI in comparison with participants with lower or normal BMI. Furthermore, a significant relationship was noted between occupational status and the occurrence of lumbar pathologies as occupations with poor ergonomics showed a higher prevalence of these lumbar pathologies.
MRI findings of lumbar pathologies such as disc changes, spondylosis, facet joint hypertrophy, neural foraminal narrowing, spinal canal stenosis etc. in these participants correlated significantly with their demographic indices (such as age, sex, occupation), BMI, LMM CSA and fatty infiltration. Participants with increasing age, BMI and LMM fatty infiltration had more lumbar pathologies than other participants. Participants with reduced LMM CSA, female gender and occupations with poor ergonomics were noted to also have more lumbar pathologies.
Paraspinal muscle morphology should be considered together with the other lumbar pathologies seen on MRI examination of the lumbar spine in individuals being investigated for CLBP and measures aimed at improving and maintaining the quality of the LMM should be encouraged, as this will help in reducing the prevalence of nonspecific CLBP.
REFERENCES
1. Doualla M, Aminde J, Aminde LN, Lekpa FK, Kwedi FM, Yenshu EV, et al. Factors influencing disability in patients with chronic low back pain attending a tertiary hospital in sub-saharan africa. BMC Musculoskeletal Disorder. 2019;20(1):25.
2. Ma K, Zhuang ZG, Wang L, Liu X-G, Lu L-J, Yang X-Q, et al. The Chinese associa-tion for the study of pain (CASP): Consensus on the Assessment and Management of Chronic Nonspecific Low Back Pain”. Pain Research and Management. 2019;8957847:14:201.
3. Vos T, Allen C, Arora M, Barber RM, Bhutta ZA, Brown A, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and inju-ries, 1990–2015: A systematic analysis for the global burden of disease study 2015. The Lan-cet. 2016;388(10053):1545-1602.
4. Buchbinder R, van Tulder M, Öberg B, Costa LM, Woolf A, Schoene M, et al. Low back pain: A call for action. The Lancet. 2018;391(10137):2384-2388.
5. Igwesi-Chidobe CN, Obiekwe C, Sorinola IO, Godfrey EL. Assessing self-reported disability in a low-literate population with chronic low back pain: Cross-cultural adaptation and psychometric testing of igbo roland morris disability questionnaire. Disability and Reha-bilitation. 2019;41(8):948-957.
6. Feitosa AA, Junior EA, Sanches LG, Borba EF, Jorge LL, Halpern AS. Chronic low back pain and sick-leave: A functional magnetic resonance study. Advances in Rheumatolo-gy. 2020;60(1):1-8.
7. Aminde JA, Aminde LN, Bija MD, Lekpa FK, Kwedi FM, Yenshu EV, et al. Health-related quality of life and its determinants in patients with chronic low back pain at a tertiary hospital in Cameroon: A cross-sectional study. BMJ Open. 2020;10(10):e035445.
8. Omokhodion FO, Sanya AO. Risk factors for low back pain among office workers in Ibadan, SouthWest Nigeria. Occupational Medicine. 2003;53(4):287-289.
9. Morris LD, Daniels KJ, Ganguli B, Louw QA. An update on the prevalence of low back pain in africa: A systematic review and meta-analyses. BMC Musculoskeletal Disor-der. 2018;19(1):1-15.
10. AlMazrou SH, Elliott RA, Knaggs RD, AlAujan SS. Cost-effectiveness of pain man-agement services for chronic low back pain: a systematic review of published studies. BMC Health Services Research. 2020;20(1):1-11.
11. Wong AY, Forss KS, Jakobsson J, Schoeb V, Kumlien C, Borglin G. Older adult’s experience of chronic low back pain and its implications on their daily life: Study protocol of a systematic review of qualitative research. Systematic Review. 2018;7(1):1-6.
12. Lekpa FK, Doualla M, Singwe-Ngandeu M, Luma HN. AB0847 non-specific chronic low back pain is common in Sub-saharan Africa: A hospital-based study in cameroon. Annals of the Rheumatic Diseases. 2016; 75:1192.
13. Bello B, Bello Adebayo H. A systematic review on the prevalence of low back pain in Nigeria. Middle East Journal of Rehabilitation and Health Studies. 2017; 4(2): e45262.
14. Omokhodion FO. Low back pain in an urban population in South West Nigeria. Trop-ical Doctor. 2004;34(1):17-20.
15. Igwesi-Chidobe CN, Kitchen S, Sorinola IO, Godfrey EL. “A life of living death”: The experiences of people living with chronic low back pain in rural Nigeria. Disability and Re-habilitation. 2017;39(8):779-790.
16. Adeojo OS. Urbanization Processes and Child Breadwinner in Lagos Metropolis. Journal of Culture, Society and Development. 2017;27:30-40.
17. Rayoffor OD, Nwankwo N, Ugboma EW, Rayoffor EJ. Evaluation of magnetic reso-nance imaging findings in adult patients with nontraumatic low back pain in South-South Ni-geria. West African Journal of Radiology. 2016;23(2):64-71.
18. Adeloye D, Ige-Elegbede JO, Ezejimofor M, Owolabi EO, Ezeigwe N, Omoyele C, et al. Estimating the prevalence of overweight and obesity in Nigeria in 2020: a systematic re-view and meta-analysis. Annals of Medicine. 2021;53(1):495-507.
19. Ogunbode AM, Adebusoye LA, Alonge TO. Prevalence of low back pain and associ-ated risk factors amongst adult patients presenting to a nigerian family practice clinic, a hospi-tal-based study. African Journal of Primary Healthcare and Family Medicine. 2013; 5(1):1-8.
20. Goubert D, Van Oosterwijck J, Meeus M, Danneels L. Structural changes of lumbar muscles in non-specific low back pain. Pain Physician. 2016;19(7):E985-E999.
21. Radziminska A, Weber-Rajek M, Strączyńska A, Zukow W. The stabilizing system of the spine. Journal of Education, Health and Sport. 2017;7(11):67-76.
22. Omoke NI, Amaraegbulam PI. Low back pain as seen in orthopedic clinics of a Nige-rian Teaching Hospital. Nigerian Journal of Clinical Practice. 2016;19(2):212-217.
23. Ahidjo A, Ayough SN, Nwobi IC, Garba I, Njiti MM, Abdullahi A. Common radio-graphic findings in patients with low back pain in a major Nigerian Teaching hospital. J Assoc of Radiographers of Nigeria. 2012;26:35-41.
24. Adekanmi AJ, Bello TO, Atalabi OM, Jimoh KO, Ogunseyinde OA. Magnetic reso-nance imaging of lumbosacral intervertebral discs in Nigerians with low back pain. West Afri-can Journal of Radiology. 2017;24(1):61-67.
25. Adekanmi AJ, Atalabi OM, Bello TO, Ogunseyinde OA. Magnetic resonance imaging pathological evidence in patients with low back pain in SouthWest Nigeria. Journal of the West African College of Surgeons. 2018;8(1):62-90.
26. Adegoke BO, Odole AC, Adeyinka AA. Adolescent low back pain among secondary school students in Ibadan, Nigeria. African Health Sciences. 2015;15(2):429-437.
27. Iyidobi EC, Obande BO, Ekwunife RT. Pattern of MRI Findings in Patients with Low Back Pain at National Orthopaedic Hospital, Enugu Nigeria. Journal of Biosciences and Medi-cines. 2018;6(4):85-94.
28. Awosan KJ, Yikawe SS, Oche OM, Oboirien M. Prevalence, perception and correlates of low back pain among healthcare workers in tertiary health institutions in Sokoto, Nigeria. Ghana Medical Journal. 2017;51(4):164-174.
29. Abdulmujeeb A, Olaniyan L. Prevalence and factors associated with low back pain among healthcare workers in Kibuli Muslim Hospital Kampala, Uganda. Epidemiology (Sunnyvale). 2017;7(1):1-5.
30. Mäki T, Oura P, Paananen M, Niinimäki J, Karppinen J, Junno J. Longitudinal analy-sis of paraspinal muscle cross-sectional area during early adulthood–a 10-year follow-up mri study. Scientific Reports. 2019;9(1):1-8.
31. Pani S, Bal NC. Aging in muscle. Models, molecules and mechanisms in biogerontolo-gy. Springer Singapore. 2020:319-345.
32. Crawford RJ, Filli L, Elliott J, Nanz D, Fischer M, Marcon M, et al. Age-and level-dependence of fatty infiltration in lumbar paravertebral muscles of healthy volunteers. Amer-ican Journal of Neuroradiology. 2016;37(4):742-748.
33. Urrutia J, Besa P, Lobos D, Andia M, Arrieta C, Uribe S. Is a single-level measurement of paraspinal muscle fat infiltration and cross-sectional area representative of the entire lum-bar spine? Skeletal Radiology. 2018;47(7):939-945.
34. Rummens S, Robben E, De Groef A, Van Wambeke P, Janssens L, Brumagne S, et al. Factors associated with the ultrasound characteristics of the lumbar multifidus: A Systematic Review. Physical Medicine and Rehabilitation. 2020;12(1):82-100.
35. Evanson AS, Myrer JW, Eggett DL, Mitchell UH, Johnson AW. Multifidus muscle size and symmetry in ballroom dancers with and without low back pain. International Journal of Sports Medicine. 2018;39(8):630-635.
36. Cuellar W, Wilson A, Blizzard CL, Otahal P, Callisaya M, Jones G, et al. The assess-ment of abdominal and multifidus muscles and their role in physical function in older adults: A systematic review. Physiotherapy. 2017;103:21-39.
37. Kalichman L, Carmeli E, Been E. The association between imaging parameters of the paraspinal muscles, spinal degeneration, and low back pain. Biomedical Research Internation-al. 2017; 2017:2-15.
38. Vohra P, Kasana VP, Arya RK. Role of MRI and ultrasonography in evaluation of multifidus muscle in chronic low back pain patients.International Journal of Research in Med-ical Sciences. 2016;4(12):5302-5309.
39. Shao X, Chen J, Yang J, Sui W, Deng Y, Huang Z, et al. Fiber type-specific morpho-logical and cellular changes of paraspinal muscles in patients with severe adolescent idio-pathic scoliosis. Medical Science Monitor. 2020;26: e924415-1–e924415-10.
40. Battaglia PJ, Maeda Y, Welk A, Hough B, Kettner N. Reliability of the Goutallier clas-sification in quantifying muscle fatty degeneration in the lumbar multifidus using magnetic resonance imaging. Journal of Manipulative and Physiological Therapeutics. 2014;37(3):190-197.
41. Hamrick MW, McGee-Lawrence ME, Frechette DM. Fatty infiltration of skeletal mus-cle: Mechanisms and comparisons with bone marrow adiposity. Frontiers in Endocrinology. 2016;7:69.
42. Wáng YX, Wáng JQ, Káplár Z. Increased low back pain prevalence in females than in males after menopause age: evidences based on synthetic literature review. Quantitative Imaging in Medicine and Surgery. 2016;6(2):199-206.
CONFLICT OF INTEREST
Authors declared no conflict of interest.
FUNDING
None.
AUTHORSHIP CONTRIBUTION
Conceptualization: MaryJane Amadi, Nengi Alazigha, Olukunmi Ijeruh, Rufus Abam, Awajimijan Nathaniel Mbaba, Michael Promise Ogolodom.
Research: MaryJane Amadi, Nengi Alazigha, Olukunmi Ijeruh, Rufus Abam, Awajimijan Nathaniel Mbaba, Michael Promise Ogolodom.
Formal analysis: MaryJane Amadi, Nengi Alazigha, Olukunmi Ijeruh, Rufus Abam, Awajimijan Nathaniel Mbaba, Michael Promise Ogolodom.
Redaction – original draft: MaryJane Amadi, Nengi Alazigha, Olukunmi Ijeruh, Rufus Abam, Awajimijan Nathaniel Mbaba, Michael Promise Ogolodom.
Redaction – proofreading and editing: MaryJane Amadi, Nengi Alazigha, Olukunmi Ijeruh, Rufus Abam, Awajimijan Nathaniel Mbaba, Michael Promise Ogolodom.