Cavitated pneumonia due to multiresistant germ in chronic kidney patient: pulmonary susceptibility and uremia
Neumonía cavitada por germen multirresistente en paciente renal crónico: susceptibilidad pulmonar y uremia
Yangel Fuentes Milián1 *![]() , Danyer Daniel Tamayo
Ribeaux2 *
, Danyer Daniel Tamayo
Ribeaux2 *![]() , Enzo Ernesto
Calderin Guzman3 *
, Enzo Ernesto
Calderin Guzman3 *![]() , Patricia Prieto
Milián4
*
, Patricia Prieto
Milián4
*![]()
1Universidad de Ciencias Médicas de Pinar del Río. Facultad de Ciencias Médicas “Dr. Ernesto Che Guevara de la Serna”. Servicio de Nefrología. Hospital General Docente “Abel Santamaría Cuadrado”. Pinar del Río. Cuba.
2Universidad de Ciencias Médicas de Granma. Facultad de Ciencias Médicas “Celia Sánchez Manduley”. Servicio de Medicina Interna. Hospital Clínico-Quirúrgico Docente “Celia Sánchez Manduley”. Granma. Cuba.
3Universidad de Ciencias Médicas de Pinar del Río. Facultad de Ciencias Médicas “Dr. Ernesto Che Guevara de la Serna”. Servicio de Medicina Interna. Hospital General Docente “Abel Santamaría Cuadrado”. Pinar del Río. Cuba.
4Universidad de Ciencias Médicas de Pinar del Río. Facultad de Ciencias Médicas “Dr. Ernesto Che Guevara de la Serna”. Pinar del Río. Cuba.
![]()
Received: 05-08-2023 Revised: 04-11-2023 Accepted: 04-02-2024 Published: 05-02-2024
How to Cite: Fuentes Milián Y, Tamayo Ribeaux DD, Calderin Guzman EE, Prieto Milián P. Cavitated pneumonia due to multiresistant germ in chronic kidney patient: pulmonary susceptibility and uremia. Interamerican Journal of Health Sciences. 2024;4:177. https://doi.org/10.59471/ijhsc2024177
ABSTRACT
Introduction: chronic kidney patients are more likely to develop infectious processes such as pneumonia, which can be caused by various microorganisms, often with atypical and multi-resistant presentations.
Objective: to present the case of a chronic kidney patient with cavitated pneumonia due to a multiresistant germ and review of the literature.
Conclusions: preventive strategies should be considered aimed at stopping antimicrobial resistance that include early diagnosis by controlling the microbiological map and make it possible to guide effective empirical treatment.
KEYWORDS
Cavitated Pneumonia, Multidrug Resistance, Chronic Renal Failure.
RESUMEN
Introducción: los pacientes renales crónicos son más propensos a desarrollar procesos infecciosos como neumonías, las que pueden ser causadas por diversos microorganismos, muchas veces con presentaciones atípicas y multirresistentes.
Objetivo: presentar el caso de un paciente renal crónico con neumonía cavitada por un germen multirresistente y revisión de la literatura.
Conclusiones: se deben pensar estrategias preventivas encaminadas a frenar la resistencia antimicrobiana que incluyan un diagnóstico precoz mediante el control del mapa microbiológico y posibiliten orientar un tratamiento empírico eficaz.
PALABRAS CLAVES
Neumonía Cavitada, Multirresistencia, Insuficiencia Renal Crónica.
INTRODUCTION
Pneumonia is an infectious and inflammatory process of the pulmonary parenchyma, of acute evolution, caused by several microorganisms which affect the distal portion of the airways and sometimes involve the alveolar interstitium. It is characterized by fever, respiratory distress and infiltrates in the chest X-ray.(1)
In patients with chronic renal failure (CRF), the incidence of respiratory infections is increased. Among the alterations that have been described in the respiratory system, it is worth mentioning the thick mucus covering the tracheobronchial tree, the thickening of the alveolar septa, covered with hyaline membranes, the presence of intra-alveolar fibrinous exudate and the decreased pulmonary clearance of bacteria.
Thus, the altered nasopharyngeal flora, the pathological changes described in the uremic lung and a propensity to aspirate contribute to the increased incidence of pulmonary infections.
Pneumonia in these patients is usually caused by Streptococcus pneumoniae. However, other germs, such as gram-negative germs, may be implicated in its occurrence, especially in hospital-acquired pneumonia.(2)
Pneumonia in immunocompromised patients, both in plain radiology and computed tomography (CT), is expressed by three general patterns: lobar pneumonia, bronchopneumonia and interstitial-alveolar pneumonia. In addition, there is a set of associated radiological signs such as pleural effusion, nodules, air bronchogram and cavitation, evident in simple radiology and others such as opacity in “ground glass”, “tree in bud”, “halo sign”, adenopathies, etc, evident in CT, which guide towards the etiological diagnosis.(3)
The causative agents of pneumonia in chronic renal patients, favoured by the conditions of the uremic environment and the decline of immunity, frequently develop states of antimicrobial multidrug resistance, manifesting themselves as atypical forms, which worsen the prognosis and make therapeutic management difficult.
Pulmonary cavitations are often associated with secondary complications such as hemoptysis and pneumothorax and confer a poor prognosis.(4)
Epidemiological surveillance of infectious events and antibiotic resistance in these patients is essential to implement improvement plans that include prevention and control activities.(5)
This article aims to present the case of a chronic renal patient with cavitary pneumonia caused by a multidrug-resistant germ.
CASE REPORT
Male patient, 39 years old, white skin, with a personal pathological history of chronic renal failure secondary to IgA glomerulopathy, in current hemodialysis treatment. He was admitted to the Nephrology Department of the General Teaching Hospital “Abel Santamaría Cuadrado” of Pinar del Río, Cuba, with a 3 day history of fever of 38,5 oC, tremors and chills, occasional dry cough, dyspnea, flank pain and lack of appetite.
Toxic habits:
· Smoker
Positive data on physical examination:
· Vesicular murmur decreased up to 1/3 middle of right lung field, polypnea: 23 x’.
· Heart rate: 114 X’, blood pressure: 140/80 mmHg.
· Painful hepatomegaly.
· Cervical adenopathies.
Complementary examinations performed:
· Hematocrit: 0,30 L/L.
· Leucograma: leucocitos: 7 x 109 /l, polimorfonucleares: 0,63; linfocitos: 0,35; eosinófilos: 0,02.
· Erythrocyte sedimentation rate: 40 mm/h.
· Platelet count: 260 x 109 /L.
· Glycemia: 4.3 mmol/L.
· Creatinine: 764 mmol/L
· Uric acid: 376 μmol/L
· Urea: 5,8 μmol/L
· Cholesterol: 3,0 mmol/L
· - Alanine aminotransferase (ALAT): 6,2 IU.
· - Aspartate aminotransferase (ASAT): 11 IU.
· Viral serology: not reactive.
· Blood cultures: negative.
· Antigen test for Covid 19: negative.
· Sputum culture: negative.
· Abdominal ultrasound: slight hepatosplenomegaly, small amount of interassociated fluid, no intra-abdominal adenopathies.
· Chest X-ray (CXR): inflammatory lesions in BPD, thick-walled cavitated image.
· Chest computed tomography (CT): moderate right pleural effusion. Cavitated images in the anterior segment of the right lower lobe and in the left upper lobe. (Figure 1).
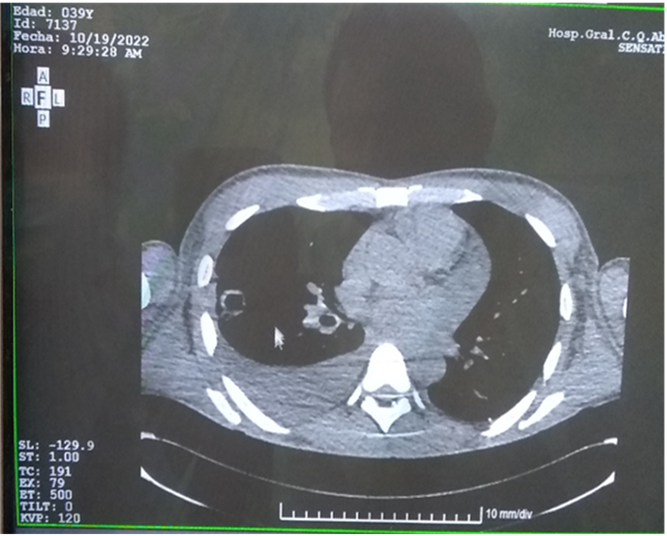
Figure 1. Chest CT: right pleural effusion and multilobular cavitated images.
Source: individual medical records.
A chest X-ray was repeated, and the signs of right hydropneumothorax and the disappearance of the cavitated lesion in the left upper lobe were shown. (Figure 2)
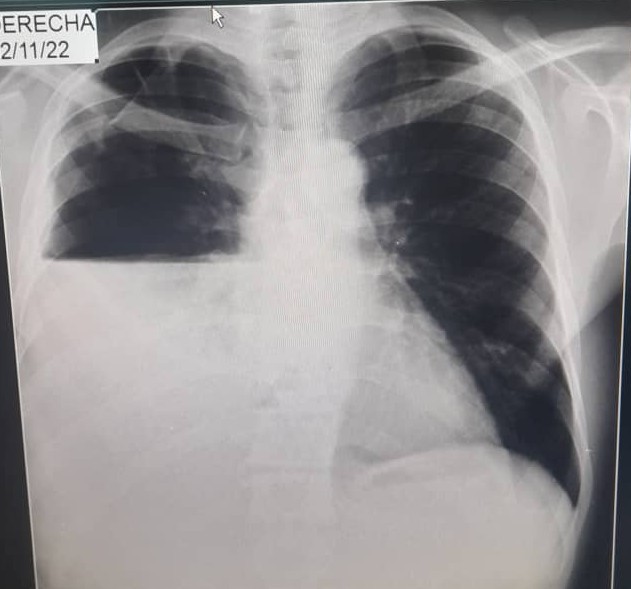
Figure 2. Chest X-ray: signs of right hydropneumothorax.
Source: individual medical records.
Patient management consisted of general measures regarding oxygen therapy, symptom control and complications. Baseline treatment was maintained.
Empirical parenteral antimicrobial treatment was started and adjusted to renal function, with ceftriaxone for ten days and vancomycin every 48 hours for five doses, without clinical or radiological improvement.
Subsequently, he was treated with meropenem for seven days without showing favourable changes in his evolution.
The patient was evaluated by the Pneumology Department, which decided to perform confirmatory studies for tuberculosis (TB) (BAAR sputum, Mantoux test, Gene-Xpert) and immediately initiate specific antibacillary treatment (isoniazid, rifampicin and pyrazinamide). This treatment caused a clinical worsening two days after initiation, accompanied by nausea and vomiting, so it was decided to discontinue it.
The results of the confirmatory studies for TB were also negative.
A thoracentesis was performed, and pleural fluid was studied, isolating the multiresistant Enterobacter agglomerans germ, sensitive to Linezolid and Ceftazidime, confirming the diagnosis of pneumonia caused by this infectious agent. Clinical and radiological improvement was evidenced two days after starting the antimicrobial treatment.
The patient remained hospitalized for 32 days, and the favourable evolution finally led to his satisfactory discharge.
DISCUSSION
A pulmonary cavity is a gas-filled space within a zone of pulmonary consolidation or a mass or nodule.
Cavities are manifestations of a wide variety of pathologic processes involving the lung and help to focus the diagnosis, as some diseases are more commonly associated with cavities than others.
From a pathologic standpoint, a cavity arises from liquefactive necrosis and subsequent expulsion of debris through the bronchial tree. The cavity wall varies in thickness depending on the underlying pathology but typically measures at least 2 mm.
The variety of infectious and noninfectious diseases associated with lung cavities can be overwhelming. However, carefully reviewing the patient’s history and radiographic data can facilitate a differential diagnostic approach. However, such studies are rarely definitive and must be complemented by focused microbiological and pathological evaluations of the affected sites, considering the possible pathogens(6)
Enterobacter strains are opportunistic pathogens that rarely cause disease in non-immunosuppressed individuals. They usually colonize hospitalized patients, particularly those receiving antibiotic treatment, diabetics, cancer patients, neutropenic patients, and patients with burns or wounds; they can also colonize the respiratory and urinary tract and intravascular catheter carriers.
Enterobacter cloacae and Enterobacter agglomerans (now Pantoea agglomerans) are the family members responsible for most hospital-acquired infections and a major epidemic resulting from perfusion contamination.(7)
P. agglomerans (formerly Enterobacter agglomerans) is a gram-negative, anaerobic, facultative, rod-shaped bacillus belonging to the family Enterobacteriaceae, which rarely causes infection in immunocompetent patients because it is an opportunistic pathogen. Its usual habitat is plants, water and animal and human faeces. It can grow in glucose-rich media, so it occasionally causes infections related to intravenous infusion of sera, which can lead to outbreaks of bacteremia in hospitals.
The germ is highly resistant to beta-lactam antibiotics, and to eradicate it, it is usually necessary to treat it according to an antibiogram.(8)
Strictly speaking, multidrug-resistant (MDR) is considered to be a microorganism that presents in vitro acquired resistance to more than one antibacterial drug. However, this MDR can be stratified in levels. The international definitions of MDR, extensively drug-resistant (XDR) and pan drug-resistant (PDR), were proposed in 2012. MDR per se is considered a microorganism that is not sensitive to at least one antimicrobial from three or more pharmaco¬logical families. The XDR is presented by the microorganism not sensitive to a drug of all categories but two or one category. PDR refers to the microorganism resistant to all drugs in all categories. Proper use of these definitions allows a better understanding of the extent of the resistance problem and makes it possible to compare epidemiological surveillance data between institutions, regions and countries.(9)
Sometimes, in practice, we still determine exactly what is most convenient. However, knowledge of infection treatment and prudent common sense can guide our activity. Until further research in larger populations, with more specific designs and more precise definitions, is carried out.(10)
CONCLUSIONS
Preventive strategies to curb antimicrobial resistance should be considered, including early diagnosis by monitoring the microbiological map and making it possible to guide effective empirical treatment.
BIBLIOGRAPHICS REFERENCES
1. Gómez Venialgo P, Torales Montiel J, Ferreira Mendieta F, Jara Rossi S, Ortega Filartiga E. Frecuencia y características clínicas de las neumonías adquiridas en la comunidad que requieren hospitalización. Rev. cient. cienc. salud [Internet]. 2020 June [cited 2023 Dec 02] ; 2( 1 ): 27-34. Available from: http://scielo.iics.una.py/scielo.php?script=sci_arttext&pid=S2664-28912020000100027&lng=en
2. Perez García R, Rodriguez Benítez P. Inmunidad e infecciones en pacientes con insuficiencia renal en hemodiálisis. En: Jofré R, López Gómez JM, Luño J, Pérez García R, Rodríguez Benítez P. Tratado de Hemodiálisis 2da ed. Barcelona: Editorial Medica Jims; 2006. p. 533-552.
3. Canals M, Sabbagh E, Chernilo S. Neumonías en el inmunocomprometido: perspectiva desde el diagnóstico por imágenes, e inferencia Bayesiana. Rev. chil. infectol. [Internet]. 2014 Abr [citado 2023 Dic 02] ; 31( 2 ): 139-152. Disponible en: http://www.scielo.cl/scielo.php?script=sci_arttext&pid=S0716-10182014000200004&lng=es. http://dx.doi.org/10.4067/S0716-10182014000200004
4. Lozano Gómez H, Herrero García S, Arche Banzo MJ, Villanueva Anadón B, Díaz Melé MC, Araiz Burdio JJ. Cavitaciones pulmonares, complicación tardía de la COVID-19. Anales Sis San Navarra [Internet]. 2022 Abr [citado 2023 Dic 02] ; 45( 1 ): e0974. Disponible en: http://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S1137-66272022000100016&lng=es
5. Andreu Périz Dolores, Hidalgo Blanco Miguel Ángel, Moreno Arroyo Carmen. Eventos infecciosos en pacientes en hemodiálisis. Enferm Nefrol [Internet]. 2015 Mar [citado 2023 Dic 06] ; 18( 1 ): 54-56. Disponible en: http://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S2254-28842015000100008&lng=es
6. Rodríguez Claudio, Vargas Bryan C., Rojas Esteban, Velásquez Carolina, de la Maza Verónica, Mancilla Edgardo. Lesiones cavitadas pulmonares: diagnóstico diferencial y revisión pictográfica. Rev. chil. radiol. [Internet]. 2023 Jun [citado 2023 Dic 02] ; 29( 2 ): 57-67. Disponible en: http://www.scielo.cl/scielo.php?script=sci_arttext&pid=S0717-93082023000200057&lng=es
7. Marcos Sánchez F., Muñoz Ruiz A. I., Martín Barranco M. J., Viana Alonso A.. Bacteriemia por Pantoea agglomerans. An. Med. Interna (Madrid) [Internet]. 2006 Mayo [citado 2023 Sep 15] ; 23( 5 ): 250-251. Disponible en: http://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S0212-71992006000500015&lng=es
8. Martín Martín Ramona. Infección del tracto urinario por Pantoea agglomerans: ¿un patógeno de pacientes inmunodeprimidos?. Rev Pediatr Aten Primaria [Internet]. 2019 Dic [citado 2023 Dic 06] ; 21( 84 ): e201-e203. Disponible en: http://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S1139-76322019000400010&lng=es
9. Camacho-Silvas Luis A., Portillo-Gallo Jorge H., Rivera-Cisneros Antonio E., Sánchez-González Jorge M., Franco-Santillán Rafael, Duque-Rodríguez Jorge et al . Multirresistencia, resistencia extendida y panresistencia a antibacterianos en el norte de México. Cir. cir. [revista en la Internet]. 2021 Ago [citado 2023 Sep 15] ; 89( 4 ): 426-434. Disponible en: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S2444-054X2021000400426&lng=es
10. Burgos Gomez Jhon A, Idoyaga Pablo, Bigot María. Neumonía cavitada y stent de la vía aérea. Rev. am. med. respir. [Internet]. 2016 Dic [citado 2023 Dic 02] ; 16( 4 ): 378-379. Disponible en: http://www.scielo.org.ar/scielo.php?script=sci_arttext&pid=S1852-236X2016000400011&lng=es
FINANCING
The authors did not receive funding for the development of this research.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORSHIP CONTRIBUTION
Conceptualization: Yangel Fuentes Milián, Danyer Daniel Tamayo Ribeaux, Enzo Ernesto Calderin Guzman, Patricia Prieto Milián.
Research: Yangel Fuentes Milián, Danyer Daniel Tamayo Ribeaux, Enzo Ernesto Calderin Guzman, Patricia Prieto Milián.
Methodology: Yangel Fuentes Milián, Danyer Daniel Tamayo Ribeaux, Enzo Ernesto Calderin Guzman, Patricia Prieto Milián.
Writing - original draft: Yangel Fuentes Milián, Danyer Daniel Tamayo Ribeaux, Enzo Ernesto Calderin Guzman, Patricia Prieto Milián.
Writing - proofreading and editing: Yangel Fuentes Milián, Danyer Daniel Tamayo Ribeaux, Enzo Ernesto Calderin Guzman, Patricia Prieto Milián.